Abstract
Acute myeloid leukemia (AML) is among the deadliest blood cancers with over 10,000 patients dying annually in the U.S. Despite high rates of complete remission after conventional frontline genotoxic chemotherapy, patient prognosis remains poor with a 5-year overall survival of only 28%, which is largely attributable to frequent relapses.
The standard chemotherapy for AML has changed little during the past 40 years. However, several new drugs have been approved by the FDA over the last 10 years including venetoclax (VEN), BH3 mimetic protein that selectively inhibits the anti-apoptotic function of BCL2. Combining VEN with the hypomethylating agent azacytidine (AZA) or with low-dose cytarabine (AraC) demonstrated promising results, leading to approval of these combinations by the FDA. However, durable response remains a challenge as resistance has emerged.
Metabolic flexibility and intratumoral heterogeneity (both genetic and non-genetic) are key components of resistance. Resistance to VEN combination therapies has implicated alternative anti-apoptosis proteins such as MCL1, monocytic differentiation state, and remodeling of mitochondrial electron transport chain complex I. TP53 or RAS mutations are also associated with resistance.
To better understand the interplay between differentiation state, metabolism, and signaling pathways in resistance to VEN and its combinations, we analyzed 36 primary AML samples (31 adult, 5 pediatric) and 2 healthy bone marrow controls using single-cell mass cytometry after ex vivo treatment for 24h with VEN, AraC, AZA and VEN+AraC and VEN+AZA. Across two antibody panels we measured myeloid phenotypic markers, key signaling pathways, transcription factors and metabolic proteins relevant to glycolysis, TCA cycle, fatty acid oxidation, amino acid transport, and mitochondrial dynamics, for a total of 76 unique markers.
For more than 80% of the patients, we observe, at baseline, highly metabolic subpopulations with high expression of ATPA5, a subunit of mitochondrial ATP synthase, and glutathione synthetase (GSS), necessary for redox homeostasis as well as strong expression of mitochondrial proteins related to oxidative phosphorylation and TCA cycle. A small subset of these cells also highly express glycolytic enzymes, in particular enolase 1 (ENO1) and lactate dehydrogenase (LDHA) which converts pyruvate to lactate. This demonstrates the presence of cells with dual glycolytic and oxidative metabolism possibly reflecting a high demand for NAD+ to support oxidation reactions. These metabolic features do not correlate with specific phenotypic markers and is highly variable between patients.
As expected, ex vivo treatment with VEN eradicates more immature subpopulations (CD34+ and/or CD117+) while sparing monocytic populations. In general, cells with the highest levels of BCL2 respond well to VEN (Panel A). However, defining populations solely with phenotypic proteins fails to adequately identify persistent and emerging populations after VEN or its combinations. Instead, clustering based on metabolic pathways identifies persistent or emerging populations. In several patients, expression of mitochondrial cristae-shaping protein (OPA1) and NADPH-producing enzymes isocitrate dehydrogenase (IDH1) or glucose-6-phosphate dehydrogenase (G6PD) defines BCL2+ subpopulations that persist through VEN treatment, likely through maintenance of mitochondrial integrity. In other patients, we observe selection of cells expressing monocarboxylate transporter 1 (MCT1), which facilitates lactate uptake, suggesting these cells undergo metabolic reprogramming to counteract the decrease in amino-acid uptakes following VEN.
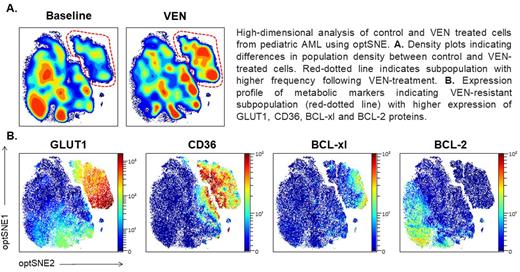
Interestingly, 4 of the 5 pediatric AML samples demonstrated VEN-resistant subpopulations characterized by co-expression of glucose transporter GLUT1, fatty acid transporter CD36, and alternative anti-apoptotic protein BCL-xL (Panel B). Very little is known about metabolic dependencies in pediatric AML and VEN is an emerging therapy in this patient group.
Altogether, our data indicate that single-cell metabolic analysis may better organize intratumoral heterogeneity in AML and enables discovery of new pathways defining resistance or persistence to VEN and its combinations. Further analyses and integration of clinical data including mutational patterns are underway and will be presented.
Disclosures
Recher:AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Jazz Pharmaceuticals: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; AbbVie, Amgen, Novartis, BMS-Celgene, Jazz Pharmaceuticals, Agios, MaatPharma, Astellas, Roche, Iqvia, Daiichi-Sankyo: Research Funding; AbbVie, Janssen, Jazz Pharmaceuticals, Novartis, BMS-Celgene, Otsuka, Astellas, Daiichi-Sankyo, Macrogenics, Roche, Takeda, Servier, Pfizer: Other: Advisory role. Sarry:Servier: Consultancy; Agios: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal